CAR-T cell therapy treatment consists of the following:
1. T Cells Collection (Leukapheresis)
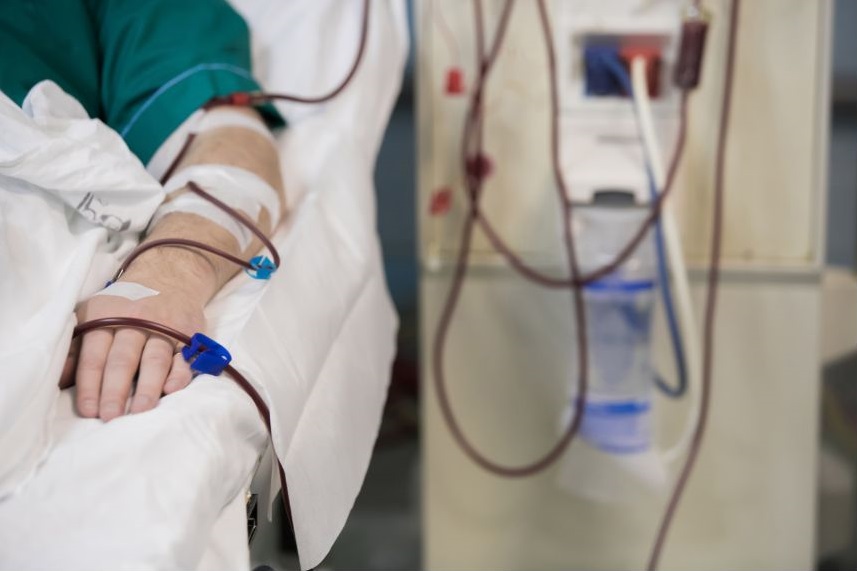
T cells will be collected from the patient by a process called leukapheresis, the patient will be connected to a machine which will extract the patient's leucocytes (white cells).
2. CAR-T Cell Manufacture

The extracted parts of the blood from leukapheresis will be sent to a laboratory where a gene for a special receptor called a chimeric antigen receptor (CAR) that binds and kills cancer cells will be added to the blood cells. The final product that is produced is the CAR-T cell.
3. Chemotherapy
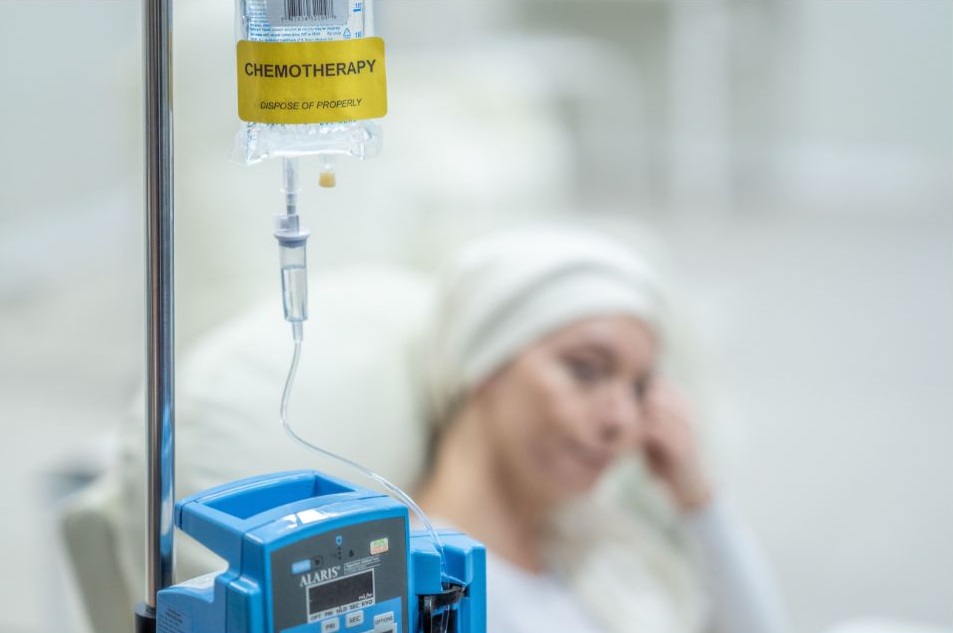
Before the CAR-T cell therapy, patients will have to undergo chemotherapy. This chemotherapy is designed to help the infused T cells to grow.
4. CAR-T Cell Infusion
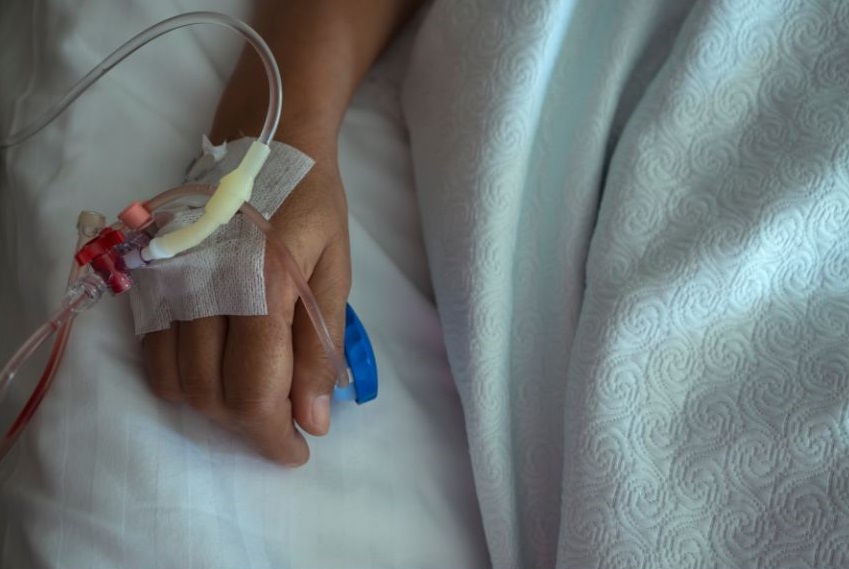
The CAR-T cells will be infused into the patient via a central catheter. Patients will be closely monitored before, during and after the treatment for the possible side effects.
Risks associated with leukapheresis
Side effects of leukapheresis include pain, nausea, fainting, dizziness, blood loss, infection, discomfort or bruising at the needle placement sites. Citrate is also usually used as a blood thinner during leukapheresis and some patients may experience muscle cramping, numbness, a cold feeling, tingling sensations or anxiety as a result of the citrate used. Post apheresis, the patient's blood counts may be lower than before the procedure. Some patients may require a transfusion of red blood cells or platelets. Patients will be closely monitored throughout the leukapheresis.
Risk of CAR-T Cell Therapy
The side effects associated with cell therapy are the main risks, however there may also be other side effects that we do not know about. There is a small risk that the patient could die from the side effects of this treatment or that the disease may continue to worsen. Finally, it is possible that even if the treatment is successful, there may also be long term side effects from CAR-T cell therapy.
The mains risks of CAR-T cell therapy are:
- Cytokine release syndrome
The infused T cells normally release chemical messengers known as cytokines to help them carry out their function. In a small percentage of cases, there may be a rapid and massive cytokine release into the bloodstream which is known as Cytokine Release Syndrome (CRS). This leads to high fevers and blood pressure drop with the risk of death, seizures, coma and multi-organ failure. The side effects can be controlled with the tociluzumab and/or the use of steroids (medication used to dampen the immune response) for the majority of CRS patients. Patients rarely develop severe symptoms which may lead to breathlessness. Intensive care support, monitoring and other immune suppression medications (other than steroids) will be rendered. If CRS occurs, it usually appears in the first 1-2 weeks after cell infusion, which warrants close monitoring.
- Immune effector cell associated neurotoxicity (ICANS)
Another side effect of the infused T cells is Immune Effector Cell Associated Neurotoxicity (ICANS) with symptoms such as confusion, seizures and fluid buildup in the brain. In severe cases, it might lead to death and severe neurological side effects. This may occur during the first 5 days or 3rd – 4th week after infusion. In view of this risk, patients are monitored closely by the physician and treated with steroids and/or tociluzumab if ICANS occurs.
Immunocompromised patients are at risk for infections, which can be life-threatening in 5% to 10% of the patients. Medicines are given to reduce this risk and most infections can be treated with antibiotics. However, viral infections cannot be treated. In some cases, patients die of infection.
- Risk related to cell processing
Small amounts of chemicals, e.g., interleukin-7 are added to the cells to help them grow outside the body. These chemicals are washed away but traces might remain. Thus there is a risk of patient's developing allergies to these materials, and there is a risk of severe reactions during or soon after the cells are infused.
There is a small risk that germs or yeast could get into the cells during processing which could cause infections after the cells are infused. This risk is small as all CAR-T cell manufacturers make every effort to keep the cells sterile (free of germs and yeast) and test the cells for these contaminants. Antibiotics and anti-yeast medicines will be given if there is any concerns about potential infections.
There is a small risk of of prolonged cytopenia (most commonly low white blood cell count and low platelet counts) post CAR-T cell therapy. This can persist even months after the treatment.
- Hypogammaglobulinaemia due to B cell aplasia
As CAR-T cell therapy affect B-cells which produce immunoglobulins, CAR-T cell therapy can result in low immunoglobulins leading to an increased risk of infections. Hypogammaglobulinaemia may last for a long time post CAR-T cell therapy. Immunoglobulin G levels will be checked periodically after CAR-T cell therapy and intravenous immunoglobulin administered if the levels are low.
